Correlation between stool form and diversity of intestinal flora among children and adolescents
-
摘要:
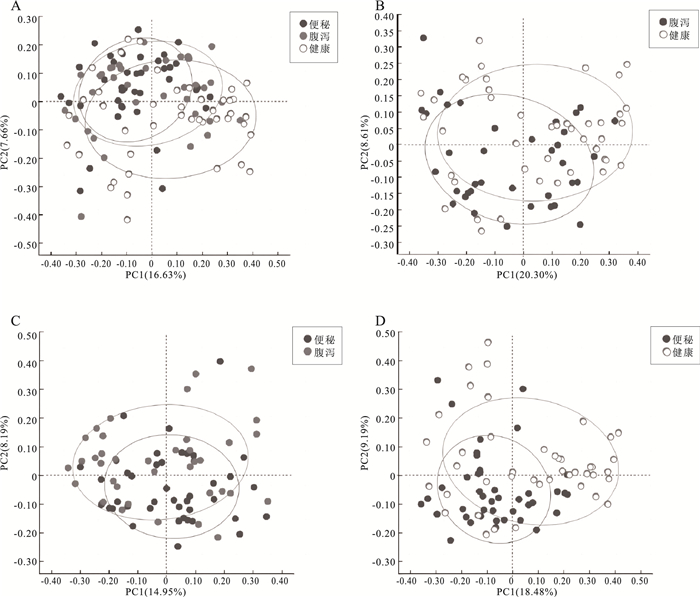
目的 研究便秘、腹泻和健康儿童青少年肠道菌群差异和多样性变化,探讨大便性状改变与肠道菌群的相关性,为儿童青少年时期的肠道微生态研究提供科学参考。 方法 2021年10月至2022年3月,采集杭州市某三甲医院收治的儿童青少年42例便秘患者、37例腹泻患者和3所普通中小学43名健康者的粪便标本,提取粪便基因组DNA进行16S rRNA基因高通量测序,对测序结果开展分析。Alpha多样性分析中,3组之间的差异比较采用Kruskal-Wallis秩和检验,两两之间比较采用FDR多重检验校正。Beta多样性分析中,3组总的差异比较采用Adonis检验方法,两两比较采用ANOSIM检验方法。LEfSe分析中,通过LDA分析(线性回归分析)获得的LDA分值。 结果 Alpha多样性分析显示,腹泻组、便秘组和健康组之间的香农(Shannon)指数(4.01,3.81,4.19)和辛普森(Simpson)指数(0.05,0.06,0.04)差异均有统计学意义(H值分别为6.05,6.35,P值均<0.1);进一步两两比较,健康组的Shannon指数和Simpson指数均高于便秘组(P值均<0.1)。Beta多样性分析显示,分组因素对组间差异的影响有统计学意义(R2=0.045,P<0.1)。群落组成分析显示,3个分组属水平上共有物种数目234个,健康组特有物种36个,腹泻组特有物种36个,便秘组特有物种48个;物种差异检验结果显示在腹泻组、便秘组和健康组之间属水平上差异有统计学意义的物种依次是厚壁菌门的毛螺菌、霍氏真杆菌、韦荣氏菌属、颤螺菌属、丁酸球菌属和葡萄球菌属(H值分别为0.000 05,0.000 16,0.000 20,0.000 21,0.000 53,0.001 39,P值均<0.1)。由KEGG丰度统计和COG功能分析可见,相同功能的基因表达丰度在不同分组间差异均无统计学意义(P值均>0.1)。 结论 儿童青少年大便性状不同与肠道微生物种群结构变化有关。与健康组相比,便秘或腹泻时肠道菌群平衡被打破,优势菌群发生偏移,条件致病菌丰度较高。 Abstract:Objective To investigate the differences and diversity changes in gut microbiota between children and adolescents with constipation and diarrhea, and healthy individuals, and to explore the correlation between changes in stool consistency and gut microbiota, in order to provide a scientific reference for the research on intestinal microecology among children and adolescents. Methods From October 2021 to March 2022, a total of 42 children and adolescents with constipation and 37 with diarrhea from a tertiary hospital in Hangzhou City, and 43 healthy individuals from 3 primary and secondary schools were included in this study. Fecal samples of children and adolescents were collected and then stool genomic DNA was extracted for 16S rRNA gene high-throughput sequencing, and the sequencing results were analyzed. In the analysis of alpha diversity, the Kruskal-Wallis rank sum test was used to compare the differences between the three groups, and the FDR multiple testing correction was used for pairwise comparisons. In the analysis of beta diversity, the Adonis test was used to compare the overall differences between the three groups, and the ANOSIM test was used for pairwise comparisons. In the LEfSe analysis, the LDA scores obtained through LDA analysis (linear regression analysis). Results Alpha diversity analysis showed that there were statistically significant differences in the Shannon index (4.01, 3.81, 4.19) and Simpson index (0.05, 0.06, 0.04) between the diarrhea group, constipation group, and healthy group (H=6.05, 6.35, P<0.1). Further pairwise comparison showed that the Shannon index and Simpson index of the healthy group were higher than those of the constipation group (P<0.1). Beta diversity analysis showed that the impact of grouping factors on inter-group differences was statistically significant (R2=0.045, P<0.1). Community composition analysis showed that there were 234 species in total among the three groups, and 36 unique species in the healthy group, 36 species in the diarrhea group, and 48 species in the constipation group. Species difference analysis showed significant differences in species composition at the genus level among the three groups (H=0.000 05, 0.000 16, 0.000 20, 0.000 21, 0.000 53, 0.001 39, P<0.1), including Lachnospiraceae of Firmicutes phylum, Eubacterium hallii, Veillonellaceae, Qscillospiraceae, Butyricicoccaceae and Staphylococcaceae, respectively. KEGG abundance statistics and COG functional analysis showed that there were no significant differences in gene expression abundance of the same function among the three groups (P>0.1). Conclusions The different stool consistency of children and adolescents is related to changes in gut microbiota composition. Compared to the healthy group, children with constipation or diarrhea have disrupted gut microbiota balance, with a shift in dominant bacteria and a higher abundance of opportunistic pathogens. -
Key words:
- Feces /
- Gastrointestinal tract /
- Bacteria /
- Child /
- Adolescent
1) 利益冲突声明 所有作者声明无利益冲突。 -
表 1 3组样本肠道菌群的LEfSe分析
Table 1. LEfSe analysis of gut murobiota among three groups
组别 微生物类群 LDA分值 便秘组 放线菌门 4.44 霍氏真杆菌 4.17 红蝽菌科 4.08 柯林斯菌属 4.08 Anaerostipes 4.02 腹泻组 消化链球菌 4.05 Peptostreptococcales-Tissierellales 4.04 罗斯氏菌 4.02 普雷沃氏菌属 3.98 韦荣球菌属 3.53 健康组 毛螺菌属 4.14 Lachnoclostridium 3.52 Butyricicoccaceae 3.51 丁酸球菌属 3.50 伯克氏菌目 3.37 表 2 3组样本肠道菌群的COG功能分类结果
Table 2. The result of COG function classification of gut microbiota among three groups
功能 健康组 便秘组 腹泻组 翻译、核糖体结构和生物发生 0.084 4 0.086 2 0.084 3 转录 0.077 8 0.079 8 0.079 4 复制、重组和修复 0.063 4 0.063 8 0.063 0 信号传导机制 0.038 4 0.038 3 0.038 4 能量生产和转换 0.059 4 0.058 8 0.059 7 碳水化合物转运和代谢 0.087 3 0.087 0 0.085 2 氨基酸转运和代谢 0.099 0 0.099 2 0.099 1 核苷酸酸转运和代谢 0.037 5 0.038 1 0.037 5 脂转运和代谢 0.023 1 0.023 3 0.023 1 无机离子转运和代谢 0.061 0 0.059 4 0.061 1 次生代谢产物的生物合成、转运和分解代谢 0.049 6 0.004 6 0.005 1 细胞运动 0.005 7 0.005 2 0.006 4 防御机制 0.027 0 0.027 3 0.026 4 -
[1] 郑德开, 张绍衡, 陈烨. 肠道菌群失衡与乳糜泻: 重要性及可能机制[J]. 微生物学报, 2021, 61(2): 292-299.ZHENG D K, ZHANG S H, CHEN Y. Dysbiosis of intestinal microbiota: key player in the pathogenesis of celiac disease[J]. Acta Microbiol Sinica, 2021, 61(2): 292-299. (in Chinese) [2] LOUIS P, SCOTT K P, DUNCAN S H, et al. Understanding the effects of diet on bacterial metabolism in the large intestine[J]. J Appl Microbiol, 2007, 102(5): 1197-1208. doi: 10.1111/j.1365-2672.2007.03322.x [3] KINROSS J M, VON ROON A C, HOLMES E, et al. The human gut microbiome: implications for future health care[J]. Curr Gastroenterol Rep, 2008, 10(4): 396-403. doi: 10.1007/s11894-008-0075-y [4] TORRAZZA R M, NEU J. The developing intestinal microbiome and its relationship to health and disease in the neonate[J]. J Perinatol, 2011, 31(S1): S29-34. doi: 10.1038/jp.2010.172 [5] HEIJTZ R D, WANG S, ANUAR F, et al. Normal gut microbiota modulates brain development and behavior[J]. Proc Natl Acad Sci USA, 2011, 108(7): 3047-3052. doi: 10.1073/pnas.1010529108 [6] FRATI F, SALVATORI C, INCORVAIA C, et al. The role of the microbiome in Asthma: the gut-lung axis[J]. Int J Mol Sci, 2018, 20(1): 123. doi: 10.3390/ijms20010123 [7] SEKIROV I, RUSSELL S L, ANTUNES L C, et al. Gut microbiota in health and disease[J]. Physiol Rev, 2010, 90(3): 859-904. doi: 10.1152/physrev.00045.2009 [8] QIN F, WU H, LI X, et al. Correlation between changes in gut flora and serum inflammatory factors in children with noninfectious diarrhea[J]. J Int Med Res, 2020, 48(1): 300060519896154. doi: 10.1177/0300060519896154 [9] 庄羽骁, 许俊, 周红艳, 等. 上海市崇明区中学生功能性便秘筛查状况[J]. 中国学校卫生, 2022, 43(9): 1391-1395. doi: 10.16835/j.cnki.1000-9817.2022.09.027ZHUANG Y X, XU J, ZHOU H Y, et al. Screening of functional constipation among adolescents in Chongming District, Shanghai[J]. Chin J Sch Health, 2022, 43(9): 1391-1395. (in Chinese) doi: 10.16835/j.cnki.1000-9817.2022.09.027 [10] 常海岭, 曾玫, 黄峥, 等. 门诊急性腹泻儿童的肠道病原监测[J]. 中华传染病杂志, 2016, 34(1): 19-22.CHANG H L, ZENG M, HUANG Z, et al. Surveillance of enteric pathogens in outpatient children with acute diarrhea[J]. Chin J Infect Dis, 2016, 34(1): 19-22. (in Chinese) [11] 伦静娴, 邹金虎, 高雪锋, 等. 广东本地及移民学龄儿童饮食结构和肠道菌群差异分析[J]. 微生物学报, 2022, 62(2): 742-753.LUN J X, ZOU J H, GAO X F, et al. Analysis of the diversity of dietary pattern and gut microbiota among local and immigrant pre-adolescent children in Guangdong[J]. Acta Microbiol Sinica, 2022, 62(2): 742-753. (in Chinese) [12] 王喜文, 郑佳, 汤漾, 等. 肠道菌群及其代谢产物对心肌纤维化影响与治疗的研究进展[J]. 微生物学报, 2023, 63(9): 3464-3481.WANG X W, ZHENG J, TANG Y, et al. Gut microbiota and its metabolites affect and help to treat myocardial fibrosis[J]. Acta Microbiol Sinica, 2023, 63(9): 3464-3481. (in Chinese) [13] WANG J, BAI X, PENG C, et al. Fermented milk containing Lactobacillus casei Zhang and bifidobacterium animalis sp. lactis V9 alleviated constipation symptoms through regulation of intestinal microbiota, inflammation, and metabolic pathways[J]. J Dairy Sci, 2020, 103(12): 11025-11038. doi: 10.3168/jds.2020-18639 [14] TING Y, YU D, DONG Q, et al. Characteristics of fecal microbiota in different constipation subtypes and association with colon physiology, lifestyle factors, and psychological status[J]. Ther Adv Gastroenterol, 2023, 16: 1-16. [15] PROCHÁZKOVÁ N, FALONY G, DRAGSTED L O, et al. Advancing human gut microbiota research by considering gut transit time[J]. Gut, 2023, 72(1): 180-191. doi: 10.1136/gutjnl-2022-328166 [16] SU T, LIU R, LEE A, et al. Altered intestinal microbiota with increased abundance of prevotella is associated with high risk of diarrhea-predominant irritable bowel syndrome[J]. Gastroenterol Res Pract, 2018, 2018: 6961783. [17] DILLON S M, LEE E J, KOTTER C V, et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection[J]. Mucosal Immunol, 2016, 9(1): 24-37. doi: 10.1038/mi.2015.33 [18] POP M, WALKER A W, PAULSON J, et al. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition[J]. Genome Biol, 2014, 27(15): R76. [19] 江学良. 益生菌制剂在消化系统中的应用[J]. 中华消化病与影像杂志, 2021, 11(2): 49-53.JIANG X L. Application of probiotics in digestive system diseases[J]. Chin J Digest Med Imageol, 2021, 11(2): 49-53. (in Chinese) [20] 汪玲娟, 张建萍, 陈慕恒. 益生菌对非酒精性脂肪肝患儿肝功能糖脂代谢及肠道菌群的影响[J]. 中国学校卫生, 2019, 40(10): 1545-1548. doi: 10.16835/j.cnki.1000-9817.2019.10.030WANG L J, ZHANG J P, CHEN M H. Effects of probiotics on liver function, glucose and lipids metabolism, fecal flora in children with nonalcoholic fatty liver disease[J]. Chin J Sch Health, 2019, 40(10): 1545-1548. (in Chinese) doi: 10.16835/j.cnki.1000-9817.2019.10.030 -
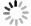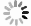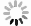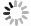






 下载:
下载: