miR-142-3p alleviates house dust mite-induced airway inflammation among children
-
摘要:
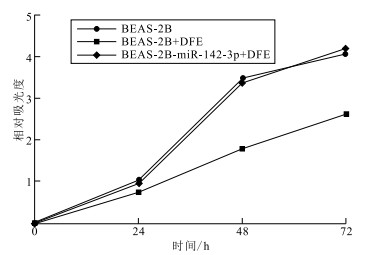
目的 探索miR-142-3p在减轻尘螨诱导的儿童过敏性气道炎症中的作用,为解析儿童过敏性气道炎症发病机制提供新思路。 方法 于2022年9—11月在江南大学附属中心医院收集15例尘螨过敏哮喘患儿和15例健康儿童血清,采用荧光定量聚合酶联反应(PCR)法检测血清中miR-142-3p表达水平。采用酶联免疫吸附试验(ELISA)法检测细胞培养上清液中炎症因子白细胞介素-6(IL-6)和肿瘤坏死因子-α(TNF-α)表达水平,采用荧光定量PCR法和Western blotting法检测高迁移率族蛋白B1(HMGB1)基因和蛋白表达水平。应用双荧光素酶报告基因系统评估miR-142-3p对HMGB1的靶向调控,采用Western blotting方法技术检测miR-142-3p干预后人正常肺上皮细胞(BEAS-2B)中下游调控蛋白表达。选择6~8周龄雌性C57BL/6小鼠,随机分为磷酸缓冲盐溶液(PBS)阴性对照组、尘螨致敏气道炎症组和尘螨致敏气道炎症+miR-142-3p干预组;采用苏木精-伊红(HE)染色评估小鼠气道炎症反应,应用瑞氏-吉姆萨染色和ELISA法检测支气管肺泡灌洗液(BALF)中炎症细胞和炎症因子表达水平。 结果 尘螨过敏哮喘患儿血清miR-142-3p表达水平较健康儿童血清降低(1.33±0.21,4.74±0.62,t=5.22,P<0.05)。粉尘螨粗提液(DFE)刺激BEAS-2B细胞后,miR-142-3p表达水平降低了(0.82±0.25);转染miR-142-3p后,其表达水平提升了(0.55±0.14)(t值分别为3.31,3.94,P值均<0.05)。miR-142-3p预处理可使DFE刺激后BEAS-2B细胞中炎症因子IL-6和TNF-α表达水平均降低(2.25±0.46,6.58±1.95)(t值分别为4.86,3.38,P值均<0.05);BEAS-2B细胞中miR-142-3p通过负调控HMGB1表达,降低下游调控蛋白Toll-样受体4蛋白(TLR4)和核因子-κB(NF-κB)表达。小鼠致敏模型显示miR-142-3p能够减轻尘螨致敏小鼠肺组织炎症细胞浸润;并且使尘螨致敏小鼠BALF中炎症因子白细胞介素-4(IL-4)、白细胞介素-5(IL-5)、HMGB1水平均降低[(107.60±10.43)pg/mL,(95.78±13.14)pg/mL,(2.52±0.87)pg/mL,t值分别为10.32,7.29,2.90,P值均<0.05]。 结论 miR-142-3p通过负调控HMGB1/TLR4/NF-κB通路减轻尘螨引起的儿童过敏性气道炎症。 Abstract:Objective To investigate the role of miR-142-3p in alleviation of house dust mite-induced allergic airway inflammation among children, so as to provide insights into unraveling the pathogenesis of allergic airway inflammation. Methods Serum samples were collected from 15 patients with house dust mite-induced allergic asthma and 15 healthy children in Jiangnan University Medical Center from September to November 2022, and serum miR-142-3p expression was quantified using a fluorescent quantitative real-time PCR (qPCR) assay. The levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were measured in the cell culture supernatant using enzyme-linked immunosorbent assay (ELISA), and the expression of high mobility group box 1 (HMGB1) was detected at transcriptional and translational lvels using qPCR and Western blotting assays. The negative regulation of the HMGB1 gene by miR-142-3p was identified using a dual luciferase gene reporter assay, and the expression of downstream regulatory proteins was determined in human normal lung epithelial cells (BEAS-2B) cells transfected with miR-142-3p using Western blotting. In addition, female C57BL/6 mice at ages of 6-8 weeks were randomly assigned to the phosphate buffer saline (PBS) group, house dust mite-sensitized airway inflammation group and house dust mite-sensitized airway inflammation + miR-142-3p intervention group. Mouse airway inflammation was evaluated using hematoxylin-eosin staining, and the expression of inflammatory cells and inflammatory cytokines were detected in mouse bronchoalveolar lavage fluid (BALF) using Giemsa staining and ELISA. Results Lower serum miR-142-3p expression was quantified among children with house dust mite-induced allergic asthma than among healthy controls (1.33±0.21 vs. 4.74±0.62, t=5.22, P < 0.05). Stimulation with dermatophagoides farinae extract (DFE) resulted in a reduction in miR-142-3p expression in BEAS-2B cells (0.82±0.25), while transfection with miR-142-3p mimics resulted in a rise in miR-142-3p expression in BEAS-2B cells (0.55±0.14)(t=3.31, 3.94, P < 0.05). Pre-treatment with miR-142-3p reduced the expression of IL-6(2.25±0.46)and TNF-α(6.58±1.95) (t=4.86, 3.38, P < 0.05) in BEAS-2B cells stimulated with DFE, and treatment with miR-142-3p mimics resulted in a reduction in TLR4 and NF-κB expression in BEAS-2B cells via negative regulation of the HMGB1 expression. In addition, treatment with miR-142-3p was found to alleviate inflammatory cell infiltration in lung tissues of house dust mite-sensitized mice, and results in a reduction in interleukin-4 (IL-4)[(107.60±10.43)pg/mL], interleukin-5 (IL-5)[(95.78±13.14)pg/mL] and HMGB1[(2.52±0.87)pg/mL] expression in BALF (t=10.32, 7.29, 2.90, P < 0.05). Conclusion miR-142-3p alleviates house dust mite-induced allergic airway inflammation among children via negative regulation of the HMGB1/TLR4/NF-κB pathway. 1) 利益冲突声明 所有作者声明无利益冲突。 -
表 1 miR-142-3p减轻DFE刺激下BEAS-2B细胞中炎症因子表达水平(x±s)
Table 1. miR-142-3p in reducing the expression of inflam- matory factors in BEAS-2B cells under DFE stimulation (x±s)
组别 miR-142-3p TNF-α IL-6 BEAS-2B 1.21±0.23 1.14±0.08 1.03±0.15 BEAS-2B+DFE 0.39±0.07 10.80±1.88 4.18±0.45 BEAS-2B-miR-142-3p+DFE 0.94±0.10 4.22±0.51 1.93±0.88 表 2 miR-142-3p减轻尘螨致敏小鼠BALF中炎症因子表达水平比较[(x±s),pg/mL]
Table 2. miR-142-3p in reducing the expression of inflammat- ory factors in BALF of house dust mite mice[(x±s), pg/mL]
组别 IL-4 IL-5 HMGB1 对照组 26.45±3.88 40.82±3.59 0.82±0.13 尘螨致敏组 173.10±8.84 144.20±11.99 8.03±0.82 尘螨致敏+miR-142-3p激动剂组 65.50±5.52 48.37±5.37 6.00±0.51 -
[1] HOLGATE S T, WENZEL S, POSTMA D S, et al. Asthma[J]. Nat Rev Dis Primers, 2015, 1(1): 15025. doi: 10.1038/nrdp.2015.25 [2] HUANG K, YANG T, XU J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study[J]. Lancet, 2019, 394(10196): 407-418. doi: 10.1016/S0140-6736(19)31147-X [3] KANAGARATHAM C, RADZIOCH D. Allergic asthma: a summary from genetic basis, mouse studies, to diagnosis and treatment[J]. Curr Pharm Des, 2016, 22(41): 6261-6272. doi: 10.2174/1381612822666160829141708 [4] THOMAS W R, HALES B J, SMITH W A. House dust mite allergens in asthma and allergy[J]. Trends Mol Med, 2010, 16(7): 321-328. doi: 10.1016/j.molmed.2010.04.008 [5] SHIPP C L, GERGEN P J, GERN J E, et al. Asthma management in children[J]. J Allergy Clin Immunol Pract, 2023, 11(1): 9-18. doi: 10.1016/j.jaip.2022.10.031 [6] GAN H, LUO W, HUANG Z, et al. House dust mite components sensitization profile in China, a multi-centre study[J]. Clin Exp Allergy, 2023, 53(2): 226-229. doi: 10.1111/cea.14255 [7] SALIMINEJAD K, KHORRAM KHORSHID H R, SOLEYMANI FA-RD S, et al. An overview of microRNAs: biology, functions, therapeutics, and analysis methods[J]. J Cell Physiol, 2019, 234(5): 5451-5465. doi: 10.1002/jcp.27486 [8] WANG X, GUO Y, WANG C, et al. microRNA-142-3p inhibits cho-ndrocyte apoptosis and inflammation in osteoarthritis by targeting HMGB1[J]. Inflammation, 2016, 39(5): 1718-1728. doi: 10.1007/s10753-016-0406-3 [9] WANG Y, LIANG J, QIN H, et al. Elevated expression of miR-142-3p is related to the pro-inflammatory function of monocyte-derived dendritic cells in SLE[J]. Arthritis Res Ther, 2016, 18(1): 263. doi: 10.1186/s13075-016-1158-z [10] WU M, HUANG Z, HUANG W, et al. microRNA-124-3p attenuates myocardial injury in sepsis via modulating SP1/HDAC4/HIF-1α axis[J]. Cell Death Discov, 2022, 8(1): 40. doi: 10.1038/s41420-021-00763-y [11] GUIOT J, CAMBIER M, BOECKX A, et al. Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p[J]. Thorax, 2020, 75(10): 870-881. doi: 10.1136/thoraxjnl-2019-214077 [12] KLEINJAN A. Airway inflammation in asthma: key players beyond the Th2 pathway[J]. Curr Opin Pulm Med, 2016, 22(1): 46-52. doi: 10.1097/MCP.0000000000000224 [13] DAVIS J D, WYPYCH T P. Cellular and functional heterogeneity of the airway epithelium[J]. Mucosal Immunol, 2021, 14(5): 978-990. doi: 10.1038/s41385-020-00370-7 [14] LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method[J]. Methods, 2001, 25(4): 402-408. doi: 10.1006/meth.2001.1262 [15] BEREDA G. Bronchial asthma: etiology, pathophysiology, diagnosis and management[J]. Austin J Pulm Respir Med, 2022, 9(1): 1085. [16] LI X, SONG P, ZHU Y, et al. The disease burden of childhood asthma in China: a systematic review and Meta-analysis[J]. J Glob Health, 2020, 10(1): 010801. doi: 10.7189/jogh.10.010801 [17] MAUER Y, TALIERCIO R M. Managing adult asthma: the 2019 GINA guidelines[J]. Cleve Clin J Med, 2020, 87(9): 569-575. doi: 10.3949/ccjm.87a.19136 [18] SIMONEAU T, CLOUTIER M M. Controversies in pediatric asthma[J]. Pediatr Ann, 2019, 48(3): e128-e134. [19] WEIDNER J, BARTEL S, KILIÇ A, et al. Spotlight on microRNAs in allergy and asthma[J]. Allergy, 2021, 76(6): 1661-1678. doi: 10.1111/all.14646 [20] QING X, ZHANG Y, PENG Y, et al. Mir-142-3p regulates inflammatory response by contributing to increased TNF-α in chronic rhinosinusitis with nasal polyposis[J]. Ear Nose Throat J, 2021, 100(1): NP50-NP56. doi: 10.1177/0145561319847972 [21] BARTEL S, CARRARO G, ALESSANDRINI F, et al. miR-142-3p is associated with aberrant WNT signaling during airway remodeling in asthma[J]. Am J Physiol Lung Cell Mol Physiol, 2018, 315(2): L328-L333. doi: 10.1152/ajplung.00113.2018 [22] WANG J Y, DONG X, YU Z, et al. Borneol inhibits CD4+ T cells proliferation by down-regulating miR-26a and miR-142-3p to attenuate asthma[J]. Int Immunopharmacol, 2021, 90: 107223. doi: 10.1016/j.intimp.2020.107223 [23] DUMITRIU I E, BARUAH P, MANFREDI A A, et al. HMGB1: an immmune odyssey[J]. Discov Med, 2005, 5(28): 388-392. [24] ZHAO Y, LI R. HMGB1 is a promising therapeutic target for asthma[J]. Cytokine, 2023, 165: 156171. doi: 10.1016/j.cyto.2023.156171 [25] KO H K, HSU W H, HSIEH C C, et al. High expression of high-mobility group box 1 in the blood and lungs is associated with the development of chronic obstructive pulmonary disease in smokers[J]. Respirology, 2014, 19(2): 253-261. doi: 10.1111/resp.12209 [26] CHENG Y, WANG D, WANG B, et al. HMGB1 translocation and release mediate cigarette smoke-induced pulmonary inflammation in mice through a TLR4/MyD88-dependent signaling pathway[J]. Mol Biol Cell, 2017, 28(1): 201-209. doi: 10.1091/mbc.e16-02-0126 [27] HUANG W F, ZHAO H J, DONG H M, et al. High-mobility group box 1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway[J]. Int J Mol Med, 2016, 37(5): 1189-1198. doi: 10.3892/ijmm.2016.2537 -






 下载:
下载: