Association between inflammation and immunity with child autism spectrum disorder based on CiteSpace analysis
-
摘要:
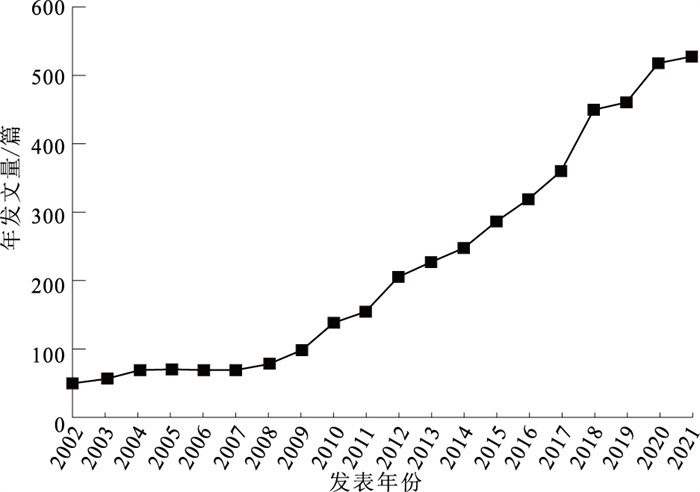
目的 对2002—2021年孤独症谱系障碍(autism spectrum disorder, ASD)炎症与免疫方面研究进行文献计量分析,为炎症与免疫因素在儿童ASD发病机制中研究提供参考。 方法 2022年5月以Web of Science数据库核心合集为文献来源,使用CiteSpace软件进行分析,最终纳入文献4 458篇。 结果 2002—2021年发文量迅速增加,由52篇增加至528篇。美国发文量最高,为2 016篇;中国次之,为407篇。高频词依次为autism spectrum disorder,children,brain,autism,spectrum disorder等,高中心性关键词依次为schizophrenia,central nervous system,mental retardation,multiple sclerosis,autoantibody等。研究趋势与热点分析表明,研究内容主要集中在核心症状及脑机制研究、基因与环境交互作用研究、流行病学研究3个方面,而母体免疫激活与肠脑轴逐渐成为研究热点。 结论 ASD炎症与免疫研究在近20年发展较为迅速,近期研究热点主要集中在母体免疫激活与肠脑轴等致病机制方面。中国应加大相关研究力度,为儿童ASD预防、干预和治疗提供科学依据。 Abstract:Objective To summarize recent progress in the inflammation and immunity research on autism spectrum disorder (ASD) in children during 2002 to 2021, and to provide future directions. Methods Literature review was conducted in May 2022, with the literature source of core collection of the Web of Science database. CiteSpace software was used for bibliometric analysis. A total of 4 458 literature was included. Results In the past decade, the number of published articles increased rapidly, from 52 to 528. American has produced the highest number of articles (n=2 016), followed by China with 407 papers published. The high-frequency words included autism spectrum disorder, children, brain, autism, spectrum disorder. The high centrality keywords included schizophrenia, central nervous system, mental regression, multiple sclerosis, autoantibody. Recent researches in ASD primarily focused on the following three aspects: core symptoms and brain mechanism, gene-environment interaction, and epidemiological research. Maternal immune activation and gut-brain axis were gradually becoming research hotspots. Conclusion Research on inflammation and immunity of ASD in children increased rapidly in the past 20 years. Recent research hotspots included maternal immune activation and gut brain axis mechanisms, which could be integreted in future efforts to develop prevention and intervention programming on ASD in children. -
Key words:
- Inflammation /
- Immunity /
- Autistic disorder /
- Mental health /
- Child
1) 利益冲突声明 所有作者声明无利益冲突。 -
表 1 ASD炎症与免疫相关研究发文量及中心性前10位国家
Table 1. The top 10 countries of the count of publications in the field of inflammation and immunity of ASD
名次 国家 首次发文年份 发文量 中心性 1 美国 2002 2 016 0.48 2 中国 2002 407 0.09 3 意大利 2002 359 0.17 4 英国 2002 301 0.12 5 日本 2002 248 0.13 6 加拿大 2003 242 0.16 7 德国 2002 182 0.22 8 法国 2002 157 0.12 9 澳大利亚 2003 144 0.04 10 沙特阿拉伯 2006 132 0.01 表 2 ASD炎症与免疫相关研究发文量及中心性前10家机构
Table 2. The top 10 institutions of the count of publications in the field of inflammation and immunity of ASD
名次 机构名称 国家 发文量 中心性 1 University of California, Davis 美国 222 0.10 2 Harvard University 美国 156 0.12 3 King Saud University 沙特阿拉伯 107 0.02 4 Johns Hopkins University 美国 92 0.08 5 Columbia University 美国 67 0.04 6 University of California, Los Angeles 美国 63 0.09 7 University of Toronto 加拿大 58 0.03 8 Tufts University 美国 54 0.02 9 University of California, San Diego 美国 47 0.02 10 King's College London 英国 46 0.02 表 3 ASD免疫与炎症相关研究高频和高中心性关键词
Table 3. High frequency and high centrality keywords in the field of inflammation and immunity of ASD
名次 高频次 高中心性 关键词 频次 关键词 中心性 1 autism spectrum disorder 948 schizophrenia 0.24 2 children 887 central nervous system 0.21 3 brain 547 mental retardation 0.21 4 autism 526 multiple sclerosis 0.20 5 spectrum disorder 470 autoantibody 0.20 6 expression 385 antibrain antibody 0.20 7 oxidative stress 301 measles virus 0.16 8 inflammation 292 nitric oxide 0.16 9 activation 284 measle 0.15 10 association 272 brain 0.14 表 4 ASD炎症与免疫相关研究关键词突现情况
Table 4. Keywords burst in the field of inflammation and immunity of ASD
关键词 突现强度 起始年份 结束年份 pervasive 29.23 2002 2012 mump 23.75 2002 2010 measle 23.61 2002 2012 immunization 19.97 2002 2012 t cell 19.97 2002 2013 inflammatory bowel disease 19.92 2002 2011 autoantibody 19.66 2002 2014 infantile autism 17.05 2002 2011 rubella vaccine 16.39 2002 2007 antibody 24.29 2003 2014 population 14.65 2003 2011 developmental disorder 13.25 2003 2009 fetal brain 15.72 2009 2014 necrosis factor alpha 15.42 2010 2014 bipolar disorder 12.26 2013 2017 prefrontal cortex 14.88 2014 2017 gut microbiota 19.35 2018 2021 neuroinflammation 13.33 2018 2019 cytokine 13.38 2019 2021 microglia 12.92 2019 2021 表 5 ASD炎症与免疫研究共被引文献突现情况
Table 5. Co-citation of literature in the field of inflammation and immunity of ASD
序号 第一作者与年份 突现强度 起始年份 结束年份 1 Vargas(2005)[4] 54.05 2005 2010 2 Ashwood(2006)[5] 37.02 2008 2011 3 Smith(2007)[6] 33.40 2009 2012 4 Li(2009)[7] 42.64 2010 2014 5 Atladóttir(2010)[8] 44.11 2011 2015 6 Ashwood(2011)[9] 41.42 2011 2016 7 Hallmayer(2011)[10] 42.50 2012 2016 8 Voineagu(2011)[11] 32.07 2012 2016 9 Onore(2012)[12] 47.20 2013 2017 10 Malkovan(2012)[13] 38.67 2013 2017 11 Hsiao(2013)[14] 51.41 2015 2018 12 American Psychiatric Association(2013)[1] 41.13 2015 2018 13 Knuesel(2014)[15] 34.09 2016 2019 14 Estes(2015)[16] 33.60 2016 2021 15 Choi(2016)[17] 53.93 2017 2021 16 Estes(2016)[18] 55.79 2018 2021 17 Kang(2017)[19] 40.51 2018 2021 18 Meltzer(2017)[20] 39.75 2018 2021 19 Kim(2017)[21] 34.82 2018 2021 20 Baio(2018)[22] 48.08 2019 2021 -
[1] American Psychiatry Association. Diagnostic and statistical manual of mental disorders[M]. 5th ed. Arlington, VA: American Psychiatric Association, 2013. [2] MAENNER M J, SHAW K A, BAKIAN A V, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2018[J]. MMWR Surveill Summ, 2021, 70(11): 1-16. doi: 10.15585/mmwr.ss7011a1 [3] ROBINSON-AGRAMONTE M L A, NORIS G E, FRAGA G J, et al. Immune dysregulation in autism spectrum disorder: what do we know about it?[J]. Inter J Molecul Sci, 2022, 23(6): 3033. doi: 10.3390/ijms23063033 [4] VARGAS D L, NASCIMBENE C, KRISHNAN C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism[J]. Ann Neurol, 2005, 57(1): 67-81. doi: 10.1002/ana.20315 [5] ASHWOOD P, WILLS S, VAN DE WATER J. The immune response in autism: a new frontier for autism research[J]. J Leukoc Biol, 2006, 80(1): 1-15. doi: 10.1189/jlb.1205707 [6] SMITH S E, LI J, GARBETT K, et al. Maternal immune activation alters fetal brain development through interleukin-6[J]. J Neurosci, 2007, 27(40): 10695-10702. doi: 10.1523/JNEUROSCI.2178-07.2007 [7] LI X, CHAUHAN A, SHEIKH A M, et al. Elevated immune response in the brain of autistic patients[J]. J Neuroimmunol, 2009, 207(1/2): 111-116. [8] ATLADÓTTIR H O, THORSEN P, ØSTERGAARD L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders[J]. J Autism Dev Disord, 2010, 40(12): 1423-1430. doi: 10.1007/s10803-010-1006-y [9] ASHWOOD P, KRAKOWIAK P, HERTZ-PICCIOTTO I, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome[J]. Brain Behav Immun, 2011, 25(1): 40-45. doi: 10.1016/j.bbi.2010.08.003 [10] HALLMAYER J, CLEVELAND S, TORRES A, et al. Genetic heritability and shared environmental factors among twin pairs with autism[J]. Arch Gen Psychiatry, 2011, 68(11): 1095-1102. doi: 10.1001/archgenpsychiatry.2011.76 [11] VOINEAGU I, WANG X, JOHNSTON P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology[J]. Nature, 2011, 474(7351): 380-384. doi: 10.1038/nature10110 [12] ONORE C, CAREAGA M, ASHWOOD P. The role of immune dysfunction in the pathophysiology of autism[J]. Brain Behav Immun, 2012, 26(3): 383-392. doi: 10.1016/j.bbi.2011.08.007 [13] MALKOVAN V, YU C Z, HSIAO E Y, et al. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism[J]. Brain Behav Immun, 2012, 26(4): 607-616. doi: 10.1016/j.bbi.2012.01.011 [14] HSIAO E Y, MCBRIDE S W, HSIEN S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders[J]. Cell, 2013, 155(7): 1451-1463. doi: 10.1016/j.cell.2013.11.024 [15] KNUESEL I, CHICHA L, BRITSCHGI M, et al. Maternal immune activation and abnormal brain development across CNS disorders[J]. Nat Rev Neurol, 2014, 10(11): 643-660. doi: 10.1038/nrneurol.2014.187 [16] ESTES M L, MCALLISTER K A. Immune mediators in the brain and peripheral tissues in autism spectrum disorder[J]. Nat Rev Neurosci, 2015, 16(8): 469-486. doi: 10.1038/nrn3978 [17] CHOI G B, YIM Y S, WONG H, et al. The maternal interleukin-17 a pathway in mice promotes autism-like phenotypes in offspring[J]. Science, 2016, 351(6276): 933-939. doi: 10.1126/science.aad0314 [18] ESTES M L, MCALLISTER A K. Maternal immune activation: implications for neuropsychiatric disorders[J]. Science, 2016, 353(6301): 772-777. doi: 10.1126/science.aag3194 [19] KANG D W, ADAMS J B, GREGORY A C, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study[J]. Microbiome, 2017, 5(1): 10. doi: 10.1186/s40168-016-0225-7 [20] MELTZER A, VAN DE WATER J. The role of the immune system in autism spectrum disorder[J]. Neuropsychopharmacology, 2017, 42(1): 284-298. doi: 10.1038/npp.2016.158 [21] KIM S, KIM H, YIM Y S, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring[J]. Nature, 2017, 549(7673): 528-532. doi: 10.1038/nature23910 [22] BAIO J, WIGGINS L, CHRISTENSEN D L, et al. Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2014[J]. MMWR Surveill Summ, 2018, 67(6): 1-23. doi: 10.15585/mmwr.ss6706a1 [23] GALEA I. The blood-brain barrier in systemic infection and inflammation[J]. Cell Mol Immunol, 2021, 18(11): 2489-2501. doi: 10.1038/s41423-021-00757-x [24] PAKHATHIRATHIEN P, JANJINDAMAI W, DISSANEEVATE S, et al. Neonatal outcomes in pregnant women with systemic lupus erythematosus: a 13-year experience in Southern Thailand[J]. J Trop Pediatr, 2021, 67(3): fmab058. doi: 10.1093/tropej/fmab058 [25] SAUNDERS N R, LIDDELOW S A, DZIEGIELEWSKA K M. Barrier mechanisms in the developing brain[J]. Front Pharmacol, 2012, 3: 46. [26] KRAKOWIAK P, GOINES P E, TANCREDI D J, et al. Neonatal cytokine profiles associated with autism spectrum disorder[J]. Biol Psychiatry, 2017, 81(5): 442-451. doi: 10.1016/j.biopsych.2015.08.007 [27] SHUID A N, JAYUSMAN P A, SHUID N, et al. Association between viral infections and risk of autistic disorder: an overview[J]. Int J Environ Res Public Health, 2021, 18(6): 2817. doi: 10.3390/ijerph18062817 [28] IVANOV Ⅱ, MCKENZIE B S, ZHOU L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells[J]. Cell, 2006, 126(6): 1121-1133. doi: 10.1016/j.cell.2006.07.035 [29] PRINS J R, ESKANDAR S, EGGEN B J L, et al. Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment?[J]. J Reprod Immunol, 2018, 126: 18-22. doi: 10.1016/j.jri.2018.01.004 [30] HAN X V, PATEL S, JONES H F, et al. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders[J]. Nat Rev Neurol, 2021, 17(9): 564-579. doi: 10.1038/s41582-021-00530-8 [31] CRYAN J F, O'RIORDAN K J, COWAN C S M, et al. The microbiota-gut-brain axis[J]. Physiol Rev, 2019, 99(4): 1877-2013. doi: 10.1152/physrev.00018.2018 [32] ZHU S, JIANG Y, XU K, et al. The progress of gut microbiome research related to brain disorders[J]. J Neur, 2020, 17(1): 25. [33] PROFACI C P, MUNJI R N, PULIDO R S, et al. The blood-brain barrier in health and disease: important unanswered questions[J]. J Exp Med, 2020, 217(4): e20190062. doi: 10.1084/jem.20190062 [34] ALAMOUDI M U, HOSIE S, SHINDLER A E, et al. Comparing the gut microbiome in autism and preclinical models: a systematic review[J]. Front Cell Infect Microbiol, 2022, 12: 905841. doi: 10.3389/fcimb.2022.905841 [35] VALENZUELA-ZAMORA A F, RAMÍREZ G-VALENZUELA D G, RAMOS-JIMÉNEZ A. Food selectivity and its implications associated with gastrointestinal disorders in children with autism spectrum disorders[J]. Nutrients, 2022, 14(13): 2660. doi: 10.3390/nu14132660 -






 下载:
下载: